
EMPOWERING TECHNOLOGY
TO INFINITE INNOVATION
INTEGRATED ENGINEERING, PROCUREMENT
AND CONSTRUCTION SOLUTION















Mechanical and plant engineering for the process industry requires fundamental insight into the respective interrelation or dependency of individual processes. Only with sound planning and construction can a plant be brought up to state-of-the-art standard.
Nowadays, nothing is more valuable to the process industry than efficient processes. And this is precisely what METECH specializes in when it comes to plant engineering. PT. Kurnia Cahaya Teknik, also known as METECH, is an Engineering, Procurement, Construction, and Commissioning (EPCC) company especially for Mechanical and Electrical.
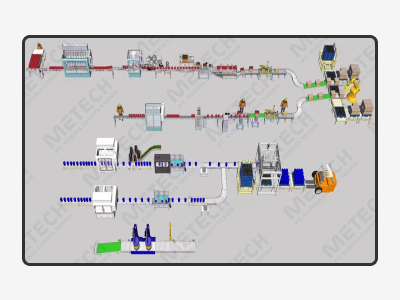
METECH provides comprehensive Mechanical, Electrical & Plumbing (MEP) engineering solutions, from basic and detailed design, through procurement and construction to commissioning and operational transfer to customers.
Our longstanding and wide-ranging project management experience ensures we consistently deliver impeccable MEP and cleanroom solutions that meet our partners’ project goals.
Our commitment to reliability extends beyond the sector of engineering services. We committed to incorporating various green engineering technologies with a life cycle perspective that involves engineering design, procurement, construction, and commissioning.
METECH offer clients economic and viable eco-friendly solutions that minimize pollution, impact on human health, and environmental damage. We furthered our commitment to sustainability by fully commitment to Cycling Efficiency and enabling continued access to resources for generations to come.




A mechanical seal has four components: a flat stationary seal face, a flat rotating seal face, a spring mechanism and a secondary elastomeric seal. The two seal faces are pressed together by a spring mechanism. The seal faces are commonly carbon, ceramic or metal. Mechanical seal applications will also incorporate an elastomer secondary seal to seal on the shaft and to seal the stationary face.
We offer a complete range of liquid and gas-lubricated pump shaft seals including standard and engineered seals in single and multi-seal versions. It is a complete range of solutions for API-682 categories and arrangements. Complete range of products compressors from a single source. Single, double, and tandem versions and tandem seals with intermedia labyrinths available. Sealing solutions for normal and sterile applications for agitators ensure the seals are rugged enough to deliver excellent cost and engineering performance in all major applicants.
Mechanical seals prevent leakage in equipment with moving parts such as mixers and pumps. It is devised to contain liquid or gas in applications where a rotating shaft goes through a stationary housing – or less commonly where the shaft is stationary, and the housing rotates around it. Any moving part creates vibration, so a mechanical seal must be precisely fitted and designed to withstand normal levels of vibration and the equipment’s ordinary movement. Fitting a mechanical seal of the optimum design, size and material can improve the lifespan and productivity of machinery. If you have any questions about our range of mechanical seals, don’t hesitate to contact us.




Static seals are an essential component in various industrial applications, including automotive, aerospace, and manufacturing. They play a critical role in creating a barrier between two surfaces that do not move relative to each other, such as the interface between a pipe and a flange or between a cylinder and a head.
One of the key benefits of static seals is their ability to provide reliable, leak-free sealing over an extended period. This is because they are made from materials that are resistant to a variety of environmental factors, such as temperature changes, pressure, and corrosive substances. Additionally, static seals are typically easy to install and require little maintenance, making them a cost-effective solution for many industrial applications.
There are several types of static seals, including gaskets, O-rings, and metal seals. Gaskets are typically made from materials such as rubber, cork, or paper, while O-rings are made from elastomers such as nitrile or silicone. Metal seals are made from materials such as copper or stainless steel and are commonly used in high-pressure or high-temperature applications.
Gaskets: Flat or profiled rings made from materials such as rubber, cork, or paper. They are commonly used in applications where a reliable, leak-free seal is required, such as engines, pumps, and pipe flanges.
O-rings: Circular rings made from elastomers such as nitrile, silicone, or Viton. They are commonly used in hydraulic and pneumatic systems, as well as in automotive and aerospace applications.
Metal seals: Typically made from materials such as copper, stainless steel, or aluminum. They are commonly used in high-temperature or high-pressure applications, such as in aerospace and power generation.
Lip seals: Designed to seal around a shaft or rod and prevent the leakage of fluids or gases. They are commonly used in engines, gearboxes, and hydraulic systems.
Diaphragms: Flexible seals that move in response to changes in pressure or fluid flow, commonly used in pumps, valves, and regulators.
Bellows seals: Accordion-like seals that can expand and contract to accommodate changes in temperature or pressure. They are commonly used in valves, pumps, and other high-pressure applications.
The choice of static seal depends on several factors, including the application requirements, the operating conditions, and the compatibility of the seal material with the media being sealed.

O-rings are used to prevent fluid or air from escaping as it acts as a sealing device. When the ring is squeezed between two surfaces, it takes up the clearance and then prevents any fluid or air from being released.
There are many different materials used for sanitary seals, and the selection may depend on the specific application and requirements. Some common materials used for sanitary seals include:


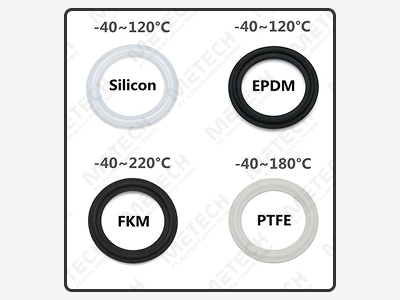


There are various approvals and certifications that sanitary seals can undergo to demonstrate their compliance with industry standards, for applications in food and beverage, pharmaceuticals, and biotechnology.
Some of the key approvals and certifications for sanitary seals are:
Face seal is a seal in which the sealing surfaces are normal to the axis of the seal. Face seals are typically used in static applications and are used to prevent leakage in the radial direction with respect to the axis of the seal. Face seals are often located in a groove or cavity on a flange.
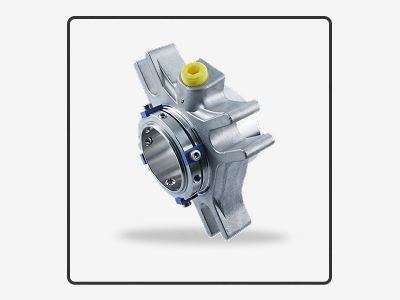
Mechanical seals can be customized to meet specific application requirements. Customization may involve selecting the appropriate materials, sizes and designs to ensure compatibility with the fluid being transferred, the operating conditions of the equipment, and the industry standards and regulations.
Customization may also involve creating specialized seals for unique applications, such as high-pressure or high-temperature environments, or for specific industries, such as the food and beverage or pharmaceutical industries, which may require specialized materials for compliance with regulations.
Mechanical seals prevent leakage in equipment with moving parts such as mixers and pumps. It is devised to contain liquid or gas in applications where a rotating shaft goes through a stationary housing – or less commonly where the shaft is stationary, and the housing rotates around it. Any moving part creates vibration, so a mechanical seal must be precisely fitted and designed to withstand normal levels of vibration and the equipment’s ordinary movement. Fitting a mechanical seal of the optimum design, size and material can improve the lifespan and productivity of machinery. If you have any questions about our range of mechanical seals, don’t hesitate to contact us.







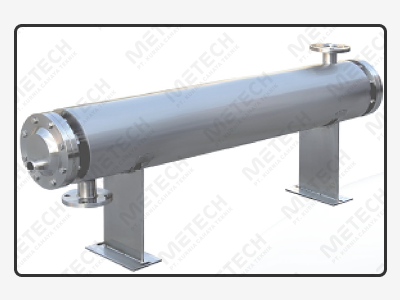
It is a high-quality Double Tube Sheet (DTS) Shell and Tube heat exchanger especially meets the high hygiene requirements in pharmaceutical industries. The heat exchanger was designed to work in the highest hygienic conditions in applications such as Water-for-Injection (WFI), Purified Water (PW), and heating / cooling pharmaceutical products.


Sanitary Tube-in-Tube heat exchanger was specially developed for WFI point of use cooling application when flow rate is less than 1500 liter / hour. It is easy to be installed and operate, quick cooling response time, no contamination risk, and free maintenance.

It is a portable cooler designed to allow samples to be taken quickly and safely at any accessible location within a clean steam or high purity steam or WFI distribution system. Cooling water connections are tri-clamp compatible which enable the unit to be connected easily to a local water supply. The whole sampler could sterilize by autoclave.
PT Kurnia Cahaya Teknik is a professional provider of lighting solutions in Indonesia. The company's team has industry experience and is an integrated clean lighting manufacturer that combines design, research and development, manufacturing and sales. It has also cooperated with well-known enterprises such as Philips and Osram and is committed to providing professional solutions in niche fields such as clean lighting. Its products are widely used in multiple lighting fields, including semiconductor factories, photovoltaic factories, industrial electronics factories, new energy, biopharmaceuticals, food, hospitals and office. The products are deeply recognized by customers.
Meanwhile, the company provides customers with comprehensive overall lighting solutions. Our business covers lighting design, lighting products, lighting construction as well as the planning, design, construction and commissioning of intelligent lighting control systems. Our mission is to strive to create the maximum value for customers. Whether customers are in the industrial market, office, or commercial outdoor lighting, we can provide them with a wide range of corresponding products, services and solutions to meet the lighting needs related to production, construction, decoration and maintenance in various fields.















| Image (click to open the image) | Project Name | Customer |
|---|---|---|
| Isolator 1 | Isolator on Filling Vial | PT. Biotis Pharmaceuticals Indonesia |
| Isolator 2 | ||
| Mixing Tank in Biotech | Mixing Tank | PT. Etana Biotechnologies Indonesia |
| Blister Machine | Blister Machine | |
| Fabrikasi Tangki | Animal Vaccine Tanks | PT. Vaksindo Satwa Nusantara |
| Sistem Transfer Emulsi | Transfer Emulsion System | |
| Extractor | Extractor System | PT. Mecosin Indonesia |
| Evaporator - Extractor | ||
| Seed Processing 1 | Seed Processing | PT. Pupuk Kujang Cikampek |
| Seed Processing 2 | ||
| Clean Room | Clean Room | PT. Nipro Indonesia Jaya |
| Plant Tissue Culture | Looping System for Plant Tissue Culture | PT. Bintang Toedjoe |
| Looping System | ||
| Pure Steam Generator | Pure Steam Generator | |
| Bioreactor | Bioreactor For Plant Tissue Culture | Universitas Surabaya |
| Waste Water Treatment | Waste Water Treatment | PT. Kino Indonesia |
| Water Treatment System | Water Treatment System | PT. Santos Jaya Abadi |
| Clean Room | Clean Room | PT. Rama Emerald Multi Sukses |
| Modifikasi Sistem Pengolahan Air | Modify Water Treatment System | PT. PIM Pharmaceuticals |
| Water Treatment System | Water Treatment System | |
| Looping PW | Looping Purified Water |











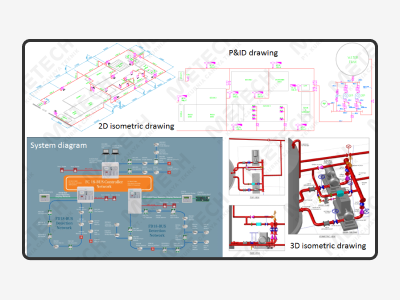
Our team provides engineering solutions across the asset life cycle. Front-end engineering and design are the work required to produce quality process and engineering documentation of sufficient depth, defining the project requirements for detailed engineering, procurement, fabrication, and construction of facilities, and supporting project cost estimate. Engineering is the heart of METECH and has been the core of the company since its established. Our engineering skills and experience are mainly based on our well understanding of technical knowledge. It is utilized at the earliest opportunity within a project, so we can add the most value and reduce the risk of change in later phases.
METECH is committed to providing security of quality, cost, and schedule, along with safe designs which are implementable and operable.
Working with clients to understand your requirements and using the learnings of hundreds of capital projects and our extensive operations portfolio, we will bring unique insights to your project. Challenging the norm, drilling into key cost drivers, and applying value-adding engineering techniques to deliver the best solutions in line with your investment strategy. Everything we do is focused on safe, fit-for-purpose design and specification, that ensures constructability and operability.
Using a flexible commercial delivery model enables us to procure directly in support of your projects. Local delivery has always been central to our approach, underpinned by robust business practices. We evaluate our impact by calculating in-country value (ICV). It is also supporting the government program about domestic component level (TKDN).
Working with local suppliers and developing local capabilities wherever possible, we ensure that all goods and services are procured in an ethical and cost-effective manner.
The success of our supply chain operations is built on several factors: our operational structure, which enables prompt approvals and decision making; our strong and long- standing relationships with our vendors; and our early commitment to long leading items through early engagement with vendors.
Our quality staff and logistic team work together to ensure that all procured items meet each project’s specific total cost, quality, and schedule requirements.
Detailed design experience means we can accurately predict requirements, supporting construction schedules through the timely availability of drawings, equipment and material and the early procurement of long lead items. This enables us to support clients in the early commencement of construction activities.
We keep costs to a minimum because we adopt 3D modeling as well as time and cost analysis in our constructability studies, which are conducted in the initial phase of an EPC project and focus on streamlining construction schedules and maximizing the efficiency of onsite workflow.
Worker safety and construction quality are paramount; we continually strive to raise safety standards in a bid to achieve zero accidents on every project we deliver. Our commitment to smart engineering extends to pioneering our proprietary HSE onsite safety and accident prevention mobile app, which significantly improves the process of identifying areas of improvement and hastens completion of daily safety systems.
Completions are key to ensuring our deliverables are safe, reliable, operable, maintainable and fit-for-purpose. Before they are handed over to you, we ensure that the facilities we design, build, and maintain are ready to be operated safely and reliably, while providing full traceability.
At no point in an EPCC project are these elements more important than in the commissioning phase, when fast and flexible communication with the project owner can make or break budgets and schedules. Our project managers ensure owners are kept fully informed of the status of the project up to the initiation of commissioning and beyond.
Our commissioning service includes critical pre-commissioning work, functional testing, and start-up operation. Our start-up teams are composed of design engineers, project engineers, construction supervisors, and operational staff with knowledge of every stage of the project. This special task force works together to ensure that every item receives proper attention during the transfer to the project owner.

PT. Etana Biotechnologies Indonesia

PT. Biotis Pharmaceuticals Indonesia

PT. Royal Haskoning Indonesia

PT. Grama Bazita

PT. Tirta Alkalindo (Eternal Plus)

PT. Mahakarya Sukses Internasional

PT. Rama Emerald Multi Sukses

PT. Cendo Pratama Farma

PT. Darya Varia Laboratoria

PT. Prafa (Pradja Pharin)

PT. Medifarma Laboratories

PT. MJB Pharma

PT. PIM Pharmaceuticals

PT. Ikapharmindo Putramas

PT. Sinde Budi Sentosa

PT. Erlangga Edi Laboratories

PT. Bintang Toedjoe

PT. Fima Internasional

PT. Pratapa Nirmala

PT. Royal Medicalink Pharmalab
PT. Mecosin Indonesia

PT. Ismut Fitomedika Indonesia

PT. Aksamala Adi Andana

PT. Pupuk Kujang

PT. Vaksindo Satwa Nusantara

PT. Kerry Ingredients Indonesia

PT. Nipro Indonesia Jaya

PT. Mane Indonesia

PT. Realfood Winta Asia




The pharmaceutical and medical device industries are subject to the most stringent quality requirements. Healthcare and patient safety always come first.
Technological advances in automation and robotics are enabling the pharmaceutical industry to increase the speed and accuracy of their processes such as filling, packaging, and inspection. Deploying automation can help pharmaceutical companies to follow stringent regulatory and compliance standards, in addition to reducing operational costs.
Automation is inevitable and ever expansive. As technology continues to advance, automation shows the potential to transform processes and introduce performance improvements. In the pharmaceutical industry, the use of automation also reduces the chance of human error through its capability to consistently perform repetitive tasks. Pharmaceutical companies have been integrating automation into specific processes like drug development, serialization, anti-counterfeiting, and more. In manufacturing examples, automation has become prevalent in processes such as kit assembly, sortation, machine tending, and packaging.
There are countless benefits to using automation and robotics in pharmaceutical development and production. Some of the most notable advantages include:
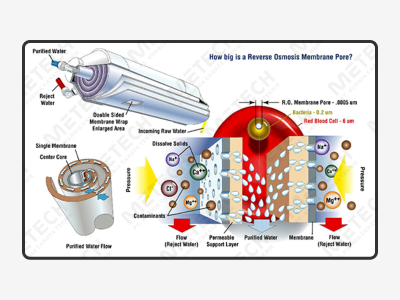
The Purified Water (PW) is mainly used for high demand water standard such as electronics, pharma, health products, food and beverages. The raw water needs to meet the drinking water standard. After filtration, Reverse Osmosis (RO), sterilization, and other processes, the Purified Water is pumped to the water point through external transfer to complete the whole preparation process. The system is made of Stainless Steel and can be effectively rust-proof. It is equipped with advanced technology of Reverse Osmosis and equipped with sterilizing devices before water point to improve water safety to meet needs of hospital, pharma industry, health products, food and IV solution production. The disinfection methods of pharma Purified Water equipment system include ozone disinfection.
The water distribution system is designed according to the actual needs of customers and disinfection requirements based on cGMP, ISPE engineering guidelines, and FDA specifications. It is with frame structure, space saving, plug and play, easy installation, pre-installation, and operation confirmation in manufacturing plant. All pipes are Stainless Steel 316L (SS-316L) with minimum grade 3A upto SF-4 with no dead angle. Sanitary connection all products can achieve temperature control, avoid bacteria breeding, and meet FDA requirements.
Pure Steam is usually steam prepared from Purified Water by Pure Steam Generator or the first effect evaporator of multi-effect distilled water machine. Both de-ionized water and Purified Water can be used as raw water of Pure Steam Generator. After evaporation, separation (removal of pollutants such as particulate and bacterial endotoxin), they can be transfer to the point of use under certain pressure.
Pharmaceutical, biomedical, and biotechnology companies face a wide range of fire hazards, in their bulk chemical facilities, clean production facilities, laboratories, warehouses and electrical infrastructure. There are also specific building requirements to eliminate collateral dangers such as fumes or substances that may be harmful to workers. These dynamic factors increase the challenges posed to fire detection and fire suppression within the industry.
The demand, therefore, is in identifying the most efficient and effective system design for each specific fire threat according to each facility requirements. Challenges include contamination of products from smoke, heat or a water sprinkler system, low temperatures, high ceilings and density of products in storage. Yet another industry issue might involve fire protection systems for the handling and dispensing of combustible liquids, solids or other hazardous materials.
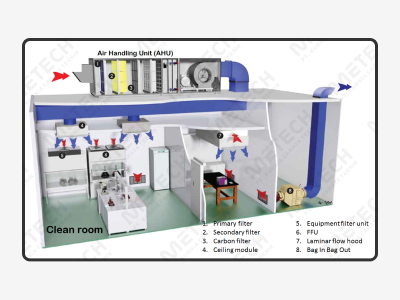
The overall solution for HVAC and clean room means blocking out and excluding all pollutants such as microparticles, harmful air, bacteria, within a certain space, reasonably distribute the overall plant structure in the area to meet the requirements of the production process. It controls the temperature, relative humidity, cleanliness, space height, room pressure, airflow velocity, airflow distribution, noise vibration, electric distribution, lighting, static electricity in the effective space within a certain area of requirements. It is a specially designed room and no matter how the external air condition exchange, the cleanliness, temperature, relative humidity, pressure, and other performance characteristics of the clean room can all maintain and ensure the original design requirements and environmental protection.
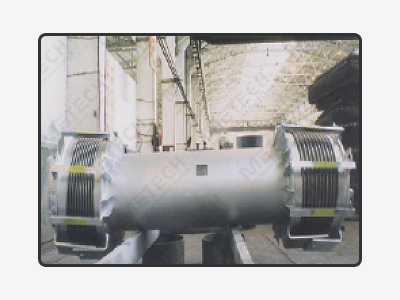
Expansion joints are vital components in most industrial plants. They are installed as flexible connections in pipe and duct systems to take up or compensate for thermal expansion, vibration, and misalignments.
Advancements in processing and generating technologies are being combined with high demands for efficiency. This, along with a clear orientation towards environmental protection, puts high demands on expansion joint designs.
A rubber expansion joint is a flexible connector to absorb noise, shock, vibration, physical and thermal energy. Made of natural or synthetic elastomers, it may be internally reinforced with fabrics and metal for strength and pressure resistance whilst metal reinforcement may be used externally for movement control.
Metal expansion joints are elastic pipe elements designed to provide compensation for expansion movements and vibrations in the pipelines. It compensates axial movements due to thermal changes compensates for torsional and angular movements, isolates vibrations, dampens noise and pressure surges.
Fabric Expansion Joints offer superior flexing capabilities compared to other solutions and can be made from a variety of special woven fabrics coated or laminated with selected elastomers or fluoropolymers. Fabric Expansion Joints are used to insulate, to avoid mechanical loads and to protect against abrasion.
They offer advantages for the pipe work designer as they can absorb movements simultaneously in several directions. Furthermore, they have almost no reactive forces and require little space. Fabric Expansion Joints are easy to customize to suit existing operating conditions and are easy to transport and install in piping and ducting systems. In comparison to Metallic Expansion Joints, Fabric Expansion Joints offer almost unlimited flexibility, giving the pipe work designer more options.
Fabric Expansion Joints are installed in systems operating with low pressure and dry media.
Cartridge seals are pre-assembled and can be easily installed into the equipment without the need for complex alignment or adjustment. They are typically more expensive than other types of seals, but they offer ease of installation, reduced downtime and improved seal performance.
Single mechanical seal consists of stationary and rotating seal faces that are made from one hard and one softer material which offers considerable cost and energy savings when used instead of gland packing.
Single Spring Mechanical Seals have single spring designs to take care of contaminated liquids. Due to open coil spring, it is used for non-clogging and self-cleaning operations. Single spring seals are assured for long-term usage and higher resistance to heat & pressure.
Single Spring Seals have very few parts and hence can be easily assembled or dismantled. The seal arrangement does not warrant any modification even in case of conversion from gland packing. Single coil Seals can be offered in Un-balance as well as Balance design to take care variety of applications.
This type of seal consists of multiple springs arranged in a circular pattern. The springs work together to create a more even pressure distribution across the seal’s faces, which can improve the seal's performance and durability. Multi-spring seals are used in a wide range of applications, including high-pressure and high-temperature environments.
A wave spring, or coiled wave spring, offers tons of advantages over other kinds of springs. The most important benefit of wave springs is that they can be half the size of traditional coiled springs. This means that the overall size and weight of an assembly can be significantly reduced. They are usually used to replace round wire compression springs in applications requiring a tight load deflection specification.
Application:
It is applicable to clean workshops and clean lighting in photovoltaic factories, semiconductor factories, precision electronics factories, pharmaceutical and medical industries, food factories, laboratories and other similar places.
| Model | MC-JJPB-12 | MC-JJPB-24 | MC-JJPB-36 | MC-JJPB-481 | MC-JJPB-482 | MC-JJPB-72 | |
|---|---|---|---|---|---|---|---|
| Size | 300 x 300 mm | 300 x 600 mm | 300 x 900 mm | 300 x 1200 mm | 600 x 600 mm | 600 x 1200 mm | |
| Voltage | 100V - 277V | ||||||
| Power | 12 W | 24 W | 36 W | 48 W | 48 W | 72 W | |
| Luminous Flux | 1200 lm | 2400 lm | 3600 lm | 4800 lm | 4800 lm | 7200 lm | |
| Color Temperature | 3000K – 6500K | ||||||
| Color Rendering Index | ≥ 80 | ||||||
| Light angle | 120° | ||||||
| Power factor | ≥ 0.9 | ||||||
| Ingress Protection | IP20 / IP54 | ||||||
| Material | Aluminum + Acrylic | ||||||
| Installation method | Crew installation or bracket installation | ||||||
| Certification | 3C/CB/IEC | ||||||
Application:
It is applicable to clean workshops and clean lighting in photovoltaic factories, semiconductor factories, precision electronics factories, pharmaceutical and medical industries, food factories, laboratories and other similar places.
| Model | MC-JJPBG-18 | MC-JJPBG-24-1 | MC-JJPBG-24-2 | MC-JJPBG-36-2 | MC-JJPBG-24-3 | MC-JJPBG-36-3 | |
|---|---|---|---|---|---|---|---|
| Size | 300 x 600 mm | 600 x 600 mm | 300 x 1200 mm | ||||
| Voltage | 100V - 277V | ||||||
| Power | 18 W | 24 W | 24 W | 36 W | 24 W | 36 W | |
| Luminous Flux | 2520 lm | 3360 lm | 3360 lm | 4800 lm | 3360 lm | 5040 lm | |
| Color Temperature | 3000K – 6500K | ||||||
| Color Rendering Index | ≥ 80 | ||||||
| Light angle | 120° | ||||||
| Power factor | ≥ 0.9 | ||||||
| Ingress Protection | IP20 / IP54 | ||||||
| Material | Aluminum + Acrylic | ||||||
| Installation method | Crew installation or bracket installation | ||||||
| Certification | 3C/CB/IEC | ||||||
Application:
Anti-ultraviolet clean flat lights can filter ultraviolet rays and are widely used in electronics factories, the semiconductor industry, photosensitive material production workshops, and chemical reagent production workshops with specific requirements for light.
| Model | MC-HGJJPB-12 | MC-HGJJPB-24 | MC-HGJJPB-36 | MC-HGJJPB-481 | MC-HGJJPB-482 | MC-HGJJPB-72 | |
|---|---|---|---|---|---|---|---|
| Size | 300 x 300 mm | 300 x 600 mm | 300 x 900 mm | 300 x 1200 mm | 600 x 600 mm | 600 x 1200 mm | |
| Voltage | 100V - 277V | ||||||
| Power | 12 W | 24 W | 36 W | 48 W | 48 W | 72 W | |
| Luminous Flux | 1200 lm | 2400 lm | 3600 lm | 4800 lm | 4800 lm | 7200 lm | |
| Color Temperature | Amber light | ||||||
| Color Rendering Index | ≥ 80 | ||||||
| Light angle | 120° | ||||||
| Power factor | ≥ 0.9 | ||||||
| Ingress Protection | IP20 / IP54 | ||||||
| Material | Aluminum + Acrylic | ||||||
| Installation method | Crew installation or bracket installation | ||||||
| Certification | 3C/CB/IEC | ||||||
Application:
It is widely used in factories, office buildings, commercial hotels, hospitals and other places.
| Model | MC-PB-12 | MC-PB-24 | MC-PB-36 | MC-PB-481 | MC-PB-482 | MC-PB-72 | |
|---|---|---|---|---|---|---|---|
| Size | 300 x 300 mm | 300 x 600 mm | 300 x 900 mm | 300 x 1200 mm | 600 x 600 mm | 600 x 1200 mm | |
| Voltage | 100V - 277V | ||||||
| Power | 12 W | 24 W | 36 W | 48 W | 48 W | 72 W | |
| Luminous Flux | 1320 lm | 2640 lm | 3960 lm | 5280 lm | 5280 lm | 7920 lm | |
| Color Temperature | 3000K – 6500K | ||||||
| Color Rendering Index | ≥ 80 | ||||||
| Light angle | 120° | ||||||
| Power factor | ≥ 0.9 | ||||||
| Ingress Protection | IP20 | ||||||
| Material | Aluminum + Acrylic | ||||||
| Installation method |
T-shaped keel embedded type
Gypsum board embedded type Aluminum buckle plate embedded type |
||||||
| Certification | 3C/CB/IEC | ||||||
Application:
It is applicable to clean workshops and clean lighting in photovoltaic factories, semiconductor factories, precision electronics factories, pharmaceutical and medical industries, food factories, and laboratories.
| Model | MC-LZ-1.2 | ||||||
|---|---|---|---|---|---|---|---|
| Size | 1225 x 50 x 80 mm | ||||||
| Voltage | 100V - 277V | ||||||
| Power | 10 W | 12 W | 14 W | 16 W | 18 W | 20 W | |
| Luminous Flux | 1200 lm | 1440 lm | 1680 lm | 1920 lm | 2160 lm | 2400 lm | |
| Color Temperature | 3000K – 6500K | ||||||
| Color Rendering Index | ≥ 80 | ||||||
| Light angle | 120° | ||||||
| Power factor | ≥ 0.9 | ||||||
| Ingress Protection | IP40 | ||||||
| Material | Aluminum + PolyCarbonate | ||||||
| Installation method | Ceiling - mounted installation | ||||||
| Certification | 3C/CB/IEC | ||||||
Application:
It is applicable to clean workshops and clean lighting in photovoltaic factories, semiconductor factories, precision electronics factories, pharmaceutical and medical industries, food factories, and laboratories.
| Model | MC-YTLZ-18 | MC-YTLZ-24 | MC-YTLZ-36 | |
|---|---|---|---|---|
| Size | 1200 mm | |||
| Voltage | 100V - 277V | |||
| Power | 18 W | 24 W | 36 W | |
| Luminous Flux | 3240 lm | 4320 lm | 6480 lm | |
| Color Temperature | 3000K – 6500K | |||
| Color Rendering Index | ≥ 80 | |||
| Light angle | 120° | |||
| Power factor | ≥ 0.9 | |||
| Ingress Protection | IP54 | |||
| Material | Poly Carbonate | |||
| Installation method |
Ceiling - mounted installation
Installation of keel |
|||
| Certification | 3C/CB/IEC | |||
Application:
It is applicable to clean workshops and clean lighting in photovoltaic factories, semiconductor factories, precision electronics factories, pharmaceutical and medical industries, food factories, and laboratories.
| Model | MC-DGJJ-1*18 | MC-SGJJ-2*18 | MC-SGJJ-3*18 | |
|---|---|---|---|---|
| Size | 1292 x 140 x 65 mm | 1292 x 210 x 65 mm | 1292 x 280 x 65 mm | |
| Quantity | 1 pcs | 2 pcs | 3 pcs | |
| Voltage | 100V - 277V | |||
| Power | 18 W x 1 | 18 W x 2 | 18 W x 3 | |
| Luminous Flux | 2160 lm | 4320 lm | 6480 lm | |
| Color Temperature | 3000K – 6500K | |||
| Color Rendering Index | ≥ 80 | |||
| Light angle | 120° | |||
| Power factor | ≥ 0.9 | |||
| Ingress Protection | IP54 | |||
| Material | Stainless steel + PolyCarbonate | |||
| Installation method | Ceiling - mounted installation | |||
| Certification | 3C/CB/IEC | |||
Application:
This kind of lighting fixture is suitable for pharmaceutical factories, food factories, electronics and semiconductor factories, biochemical industries and the cosmetics industry. It can be used in all clean rooms and clean areas.
| Model | MC-QSJ-2 | MC-QSJ-3 | |
|---|---|---|---|
| Size | 1170 x 570 x 170 mm | ||
| Voltage | 100V - 277V | ||
| Power | 18 W x 2 | 18 W x 3 | |
| Luminous Flux | 4320 lm | 6480 lm | |
| Color Temperature | 3000K – 6500K | ||
| Color Rendering Index | ≥ 80 | ||
| Light angle | 120° | ||
| Power factor | ≥ 0.9 | ||
| Ingress Protection | IP5 | ||
| Material | Cold-rolled sheet + Tempered glass | ||
| Installation method | Embedded installation | ||
| Certification | 3C/CB/IEC | ||
Application:
It is applicable to clean workshops and clean lighting in photovoltaic factories, semiconductor factories, precision electronics factories, pharmaceutical and medical industries, food factories, laboratories and other similar places.
| Model | MC-LG-8 | MC-LG-15 | MC-LG-24 | |
|---|---|---|---|---|
| Size | 495 mm | 795 mm | 1095 mm | |
| Voltage | 100V - 277V | |||
| Power | 8 W | 15 W | 24 W | |
| Luminous Flux | 960 lm | 1800 lm | 2880 lm | |
| Color Temperature | 3000K – 6500K | |||
| Color Rendering Index | ≥ 80 | |||
| Light angle | 120° | |||
| Power factor | ≥ 0.9 | |||
| Ingress Protection | IP54 | |||
| Material | Poly Carbonate | |||
| Installation method | Suspended ceiling with keel | |||
| Certification | 3C/CB/IEC | |||
Application:
Food factories, biological laboratories, operating rooms, pharmaceutical factories.
| Model | MC-SJD-18 | MC-SJD-30 | MC-SJD-36 | MC-WGSJD-18 | MC-WGSJD-30 | MC-WGSJD-36 | |
|---|---|---|---|---|---|---|---|
| Size | 620 x 60 x 90 mm | 925 x 60 x 90 mm | 1230 x 60 x 90 mm | 695 x 300 mm | 1000 x 300 mm | 1305 x 300 mm | |
| Voltage | 100V - 277V | ||||||
| Power | 18 W | 30 W | 36 W | 18 W | 30 W | 36 W | |
| Color Temperature | 3000K – 6500K | ||||||
| Color Rendering Index | ≥ 80 | ||||||
| Light angle | 120° | ||||||
| Power factor | ≥ 0.9 | ||||||
| Ingress Protection | IP20 | ||||||
| Material | Stainless steel | ||||||
| Installation method |
Ceiling - mounted installation
Fixed installation |
||||||
| Certification | 3C/CB/IEC | ||||||
Application:
Food factories, biological laboratories, operating rooms, pharmaceutical factories.
| Model | MC-GK-A-50 | MC-GK-A-100 | MC-GK-A-120 | MC-GK-A-150 | MC-GK-A-200 | |
|---|---|---|---|---|---|---|
| Size | Ø240 x H 68 mm | Ø270 x H 68 mm | ø300 x H 70 mm | ø300 x H 70 mm | ø335 x H 70 mm | |
| Voltage | 100V - 277V | |||||
| Power | 50 W | 100 W | 120 W | 150 W | 200 W | |
| Luminous Flux | 5500 lm | 11000 lm | 13200 lm | 16500 lm | 22000 lm | |
| Color Temperature | 3000K – 6500K | |||||
| Color Rendering Index | ≥ 80 | |||||
| Light angle | 120° | |||||
| Power factor | ≥ 0.9 | |||||
| Ingress Protection | IP65 | |||||
| Material | Aluminum | |||||
| Installation method | Suspension installation | |||||
| Certification | 3C/CB/IEC | |||||
Application:
It is widely used in large spaces such as factories and public places.
| Model | MC-GK-50 | MC-GK-80 | MC-GK-100 | MC-GK-120 | MC-GK-150 | MC-GK-200 | MC-GK-250 | MC-GK-300 | |
|---|---|---|---|---|---|---|---|---|---|
| Size | Φ240 x 110 mm | Φ240 x 110 mm | Φ240 x 110 mm | Φ240 x 110 mm | Φ280 x 114 mm | Φ320 x 118 mm | Φ360 x 131 mm | Φ360 x 135 mm | |
| Voltage | 100V - 277V | ||||||||
| Power | 50 W | 80 W | 100 W | 120 W | 150 W | 200 W | 250 W | 300 W | |
| Luminous Flux | 7000 lm | 11200 lm | 14000 lm | 16800 lm | 21000 lm | 28000 lm | 35000 lm | 42000 lm | |
| Color Temperature | 3000K – 6500K | ||||||||
| Color Rendering Index | ≥ 80 | ||||||||
| Light angle | 120° | ||||||||
| Power factor | ≥ 0.9 | ||||||||
| Ingress Protection | IP65 | ||||||||
| Material | Die-cast aluminum | ||||||||
| Installation method | Suspension installation | ||||||||
| Certification | 3C/CB/IEC | ||||||||
Application:
It is widely used in large spaces such as factories and public places.
| Model | MC-GKG-50 | MC-GKG-80 | MC-GKG-100 | MC-GKG-120 | MC-GKG-150 | MC-GKG-200 | MC-GKG-250 | MC-GKG-300 | |
|---|---|---|---|---|---|---|---|---|---|
| Size | Φ240 x 110 mm | Φ240 x 110 mm | Φ240 x 110 mm | Φ240 x 110 mm | Φ280 x 114 mm | Φ320 x 118 mm | Φ360 x 131 mm | Φ360 x 135 mm | |
| Voltage | 100V - 277V | ||||||||
| Power | 50 W | 80 W | 100 W | 120 W | 150 W | 200 W | 250 W | 300 W | |
| Luminous Flux | 9000 lm | 14400 lm | 18000 lm | 21600 lm | 27000 lm | 36000 lm | 45000 lm | 54000 lm | |
| Color Temperature | 3000K – 6500K | ||||||||
| Color Rendering Index | ≥ 80 | ||||||||
| Light angle | 120° | ||||||||
| Power factor | ≥ 0.9 | ||||||||
| Ingress Protection | IP65 | ||||||||
| Material | Die-cast aluminum | ||||||||
| Installation method | Suspension installation | ||||||||
| Certification | 3C/CB/IEC | ||||||||
Application:
It is widely used in large spaces like factories, gymnasiums and public places.
| Model | MC-TG-50 | MC-TG-80 | MC-TG-100 | MC-TG-120 | MC-TG-150 | MC-TG-200 | MC-TG-250 | MC-TG-300 | |
|---|---|---|---|---|---|---|---|---|---|
| Size | 155 x 185 x 45 mm | 170 x 200 x 45 mm | 200 x 230 x 55 mm | 200 x 230 x 55 mm | 255 x 280 x 65 mm | 255 x 280 x 65 mm | 310 x 345 x 70 mm | 310 x 345 x 70 mm | |
| Voltage | 100V - 277V | ||||||||
| Power | 50 W | 80 W | 100 W | 120 W | 150 W | 200 W | 250 W | 300 W | |
| Luminous Flux | 6000 lm | 9600 lm | 12000 lm | 14400 lm | 18000 lm | 24000 lm | 30000 lm | 36000 lm | |
| Color Temperature | 3000K – 6500K | ||||||||
| Color Rendering Index | ≥ 80 | ||||||||
| Light angle | 120° | ||||||||
| Power factor | ≥ 0.9 | ||||||||
| Ingress Protection | IP65 | ||||||||
| Material | Die-cast aluminum | ||||||||
| Installation method |
Suspension installation
Bracket installation |
||||||||
| Certification | 3C/CB/IEC | ||||||||
Application:
It is widely used in factories, office buildings, commercial hotels, hospitals and other places.
| Model | MC-DGZJ-18 | MC-DGZJDZ-18 | MC-SGZJ-36 | MC-SGZJDZ-36 | |
|---|---|---|---|---|---|
| Size | 1200 mm | ||||
| Voltage | 100V - 277V | ||||
| Power | 18 W x 1 | 18 W x 1 | 18 W x 2 | 18 W x 2 | |
| Luminous Flux | 2160 lm | 2160 lm | 4320 lm | 4320 lm | |
| Color Temperature | 3000K – 6500K | ||||
| Color Rendering Index | ≥ 80 | ||||
| Light angle | 120° | ||||
| Power factor | ≥ 0.9 | ||||
| Ingress Protection | IP20 | ||||
| Material | Cold-rolled sheet | ||||
| Installation method |
Ceiling - mounted installation
Suspended installation |
||||
| Certification | 3C/CB/IEC | ||||
Application:
It is widely used in lighting areas that require dustproof and waterproof functions, such as factories, office buildings, commercial hotels, and hospitals.
| Model | MC-DGSF-18 | MC-SGSF-36 | |
|---|---|---|---|
| Size | 1260 x 85 x 85 mm | 1260 x 120 x 85 mm | |
| Voltage | 100V - 277V | ||
| Power | 18 W x 1 | 18 W x 2 | |
| Luminous Flux | 2160 lm | 4320 lm | |
| Color Temperature | 3000K – 6500K | ||
| Color Rendering Index | ≥ 80 | ||
| Light angle | 120° | ||
| Power factor | ≥ 0.9 | ||
| Ingress Protection | IP65 | ||
| Material | PolyCarbonate | ||
| Installation method |
Ceiling - mounted installation
Suspended installation |
||
| Certification | 3C/CB/IEC | ||
Application:
It is widely used in lighting areas that require dustproof and waterproof functions, such as factories, office buildings, commercial hotels, and hospitals.
| Model | MC-YTSF1-18 | MC-YTSF1-24 | MC-YTSF2-36 | MC-YTSF2-48 | |
|---|---|---|---|---|---|
| Voltage | 100V - 277V | ||||
| Power | 18 W | 24 W | 36 W | 48 W | |
| Luminous Flux | 2160 lm | 2880 lm | 4320 lm | 5760 lm | |
| Color Temperature | 3000K – 6500K | ||||
| Color Rendering Index | ≥ 80 | ||||
| Light angle | 120° | ||||
| Power factor | ≥ 0.9 | ||||
| Ingress Protection | IP65 | ||||
| Material | PolyCarbonate + Acrylic | ||||
| Installation method | Ceiling - mounted installation | ||||
| Certification | 3C/CB/IEC | ||||
Application:
It is applicable to photovoltaic factories, semiconductor factories, electronics factories, pharmaceutical and food factories, laboratories, and chemical factory workshops.
| Model | MC-NGWZJ-24 | MC-NGWZJ-36 | MC-NGWZJ-48 | |
|---|---|---|---|---|
| Size | 120 x 118 x 90 mm | |||
| Voltage | 100V - 277V | |||
| Power | 24 W | 36 W | 48 W | |
| Luminous Flux | 2400 lm | 3600 lm | 4800 lm | |
| Color Temperature | 3000K – 6500K | |||
| Color Rendering Index | ≥ 80 | |||
| Light angle | 120° | |||
| Power factor | ≥ 0.9 | |||
| Ingress Protection | Heat - resistant temperature: 85℃ | |||
| Material | Stainless steel + tempered glass | |||
| Installation method |
Ceiling - mounted installation
Suspended installation |
|||
| Certification | 3C/CB/IEC | |||
Application:
Explosive gas hazardous areas, chemical factories, fireworks and firecracker factories, mining and excavation operations, powder dust and other environments.
| Model | MC-FB-DG18 | MC-FB-SG36 | |
|---|---|---|---|
| Size | 1200 mm | ||
| Voltage | 100V - 277V | ||
| Power | 18 W x 1 | 18 W x 2 | |
| Luminous Flux | 2160 lm | 4320 lm | |
| Color Temperature | 3000K – 6500K | ||
| Color Rendering Index | ≥ 80 | ||
| Light angle | 120° | ||
| Power factor | ≥ 0.9 | ||
| Ingress Protection | Ex d IIB T6 GB/Ex tD a21 IP65 T80℃ | ||
| Material | Aluminum Alloy + Tempered Glass | ||
| Installation method | Ceiling - mounted installation | ||
| Certification | 3C/CB/IEC | ||
Application:
Bedrooms, hotels, bathrooms, offices, shopping malls.
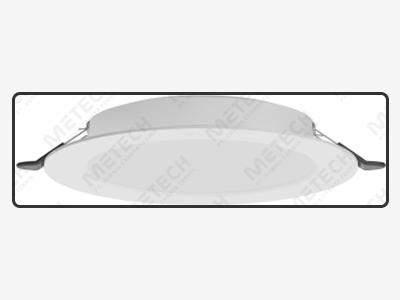
| Model | MC-XD-12 | MC-XD-18 | MC-XD-24 | |
|---|---|---|---|---|
| Size | Φ273 x 60 | Φ320 x 60 | Φ375 x 68 | |
| Voltage | 100V - 277V | |||
| Power | 12 W | 18 W | 24 W | |
| Luminous Flux | 960 lm | 1440 lm | 1920 lm | |
| Color Temperature | 3000K – 6500K | |||
| Color Rendering Index | ≥ 80 | |||
| Light angle | 120° | |||
| Power factor | ≥ 0.9 | |||
| Ingress Protection | IP20 | |||
| Material | PolyCarbonate | |||
| Installation method | Ceiling - mounted installation | |||
| Certification | 3C/CB/IEC | |||
Application:
Bedrooms, hotels, bathrooms, offices, shopping malls.
| Model | MC-TD-8 | MC-TD-12 | MC-TD-16 | MC-TD-20 | MC-TD-24 | |
|---|---|---|---|---|---|---|
| Size | Φ100 | Φ110 | Φ130 | Φ165 | Φ210 | |
| Voltage | 100V - 277V | |||||
| Power | 8 W | 12 W | 16 W | 20 W | 24 W | |
| Luminous Flux | 640 lm | 960 lm | 1280 lm | 1600 lm | 1920 lm | |
| Color Temperature | 3000K – 6500K | |||||
| Color Rendering Index | ≥ 80 | |||||
| Light angle | 120° | |||||
| Power factor | ≥ 0.9 | |||||
| Ingress Protection | IP20 | |||||
| Material | PolyCarbonate | |||||
| Installation method | Embedded | |||||
| Certification | 3C/CB/IEC | |||||
Application:
Bedrooms, hotels, bathrooms, offices, shopping malls.
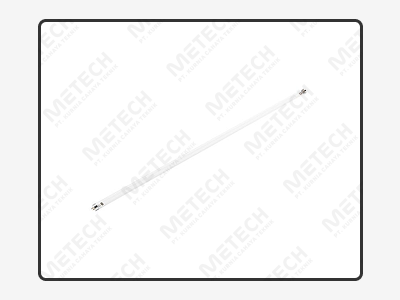
| Model | MC-TD-8 | |||
|---|---|---|---|---|
| Size | 8 x 1.2 mm | |||
| Voltage | DC 24 V | |||
| Power | 4.8 W | 8 W | 12 W | |
| Luminous Flux | 480 lm | 800 lm | 1200 lm | |
| Color Temperature | 2700K – 6500K | |||
| Color Rendering Index | ≥ 90 | |||
| Light angle | 120° | |||
| Power factor | ≥ 0.9 | |||
| Ingress Protection | IP20 | |||
| Material | FPC | |||
| Installation method | Back sticker | |||
| Certification | 3C/CB/IEC | |||
Application:
Bedrooms, hotels, bathrooms, offices, shopping malls.
| Model | MC-DYDD2 | |||
|---|---|---|---|---|
| Size | 8 x 1.2 mm | |||
| Voltage | DC 24 V | |||
| Power | 4.8 W | 8 W | 12 W | |
| Luminous Flux | 480 lm | 800 lm | 1200 lm | |
| Color Temperature | 2700K – 6500K | |||
| Color Rendering Index | ≥ 90 | |||
| Light angle | 120° | |||
| Power factor | ≥ 0.9 | |||
| Ingress Protection | IP65 | |||
| Material | FPC | |||
| Installation method | Installation by Snap Fasteners | |||
| Certification | 3C/CB/IEC | |||
Application:
It is applicable to offices, shopping malls, hotels, factory workshops, warehouses.
| Model | MC-DG-10 | MC-DG-12 | MC-DG-16 | MC-DG-18 | MC-DG-20 | |
|---|---|---|---|---|---|---|
| Size | φ26 x 1.2 mm | |||||
| Voltage | 100 V – 277 V | |||||
| Power | 10 W | 12 W | 16 W | 18 W | 20 W | |
| Luminous Flux | 1800 lm | 2160 lm | 2880 lm | 3240 lm | 3600 lm | |
| Color Temperature | 2700K – 6500K | |||||
| Color Rendering Index | ≥ 90 | |||||
| Light angle | 120° | |||||
| Power factor | ≥ 0.9 | |||||
| Ingress Protection | IP20 | |||||
| Material | Plastic + glass | |||||
| Installation method | Installation by Snap Fasteners | |||||
| Certification | 3C/CB/IEC | |||||